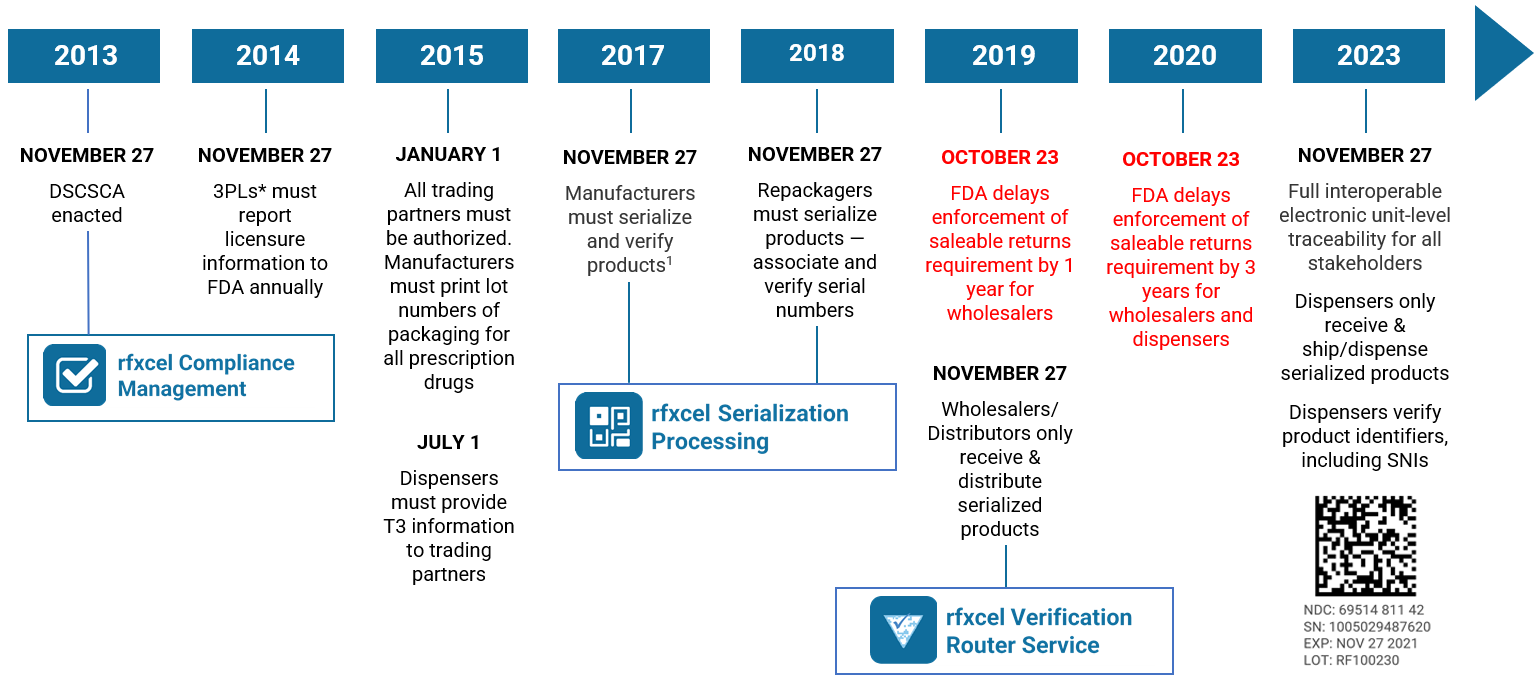
Welcome to Part II of our brand protection series. In Part I, we talked about the top supply chain threats that brand protection strategies must address: counterfeits, diversion, theft, and insufficient traceability.
Today, we’re talking about the supply chain solutions that combat these threats — solutions that should be integral to your brand protection strategies.
A holistic supply chain approach to brand protection strategies
Your supply chain is the embodiment of your business. If it’s not healthy, your brand can’t be healthy. You need to be able to continuously scan it, diagnose it, and take immediate action should a threat arise.
Fine-tuned digital supply chain solutions are the answer. Working together from end to end, they create a “central nervous system” that monitors and senses every touch point in real time while collecting and sharing data to inform your next move.
Serialization, real-time monitoring, and end-to-end traceability are the key solutions that help mitigate supply chain threats and provide the intelligence for effective brand protection strategies. Let’s take a look.
Serialization
Serialization is the building block of a secure supply chain and effective brand protection strategies. By assigning a unique serial number to each product, you create what rfxcel calls a “digital asset” with a unique digital identity. Every product can be linked to virtually any information you want, such as its origin, when it was harvested or manufactured, its lot number, and its expiration date.
Serialization also enables you to track every individual unit through your entire supply chain, from production to retail distribution to the final consumer and beyond. It creates a barrier to fight counterfeits and fakes and contributes to end-to-end traceability that eliminates blind spots and locks down your supply chain.
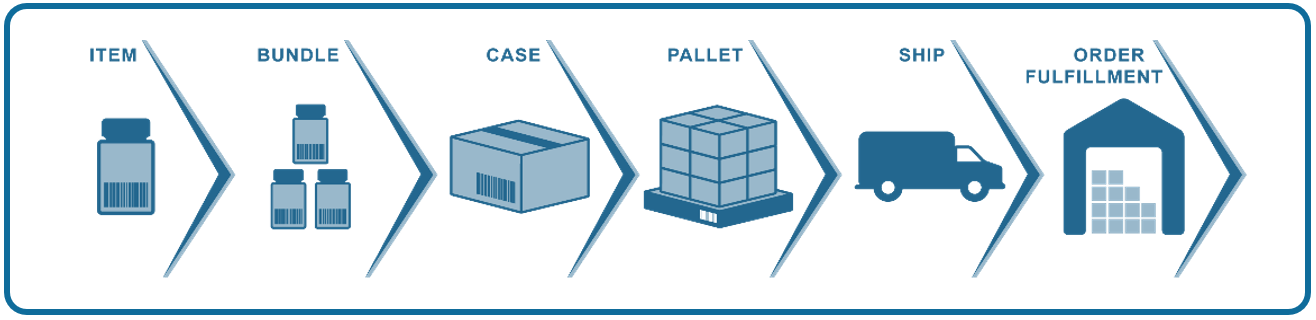
Serialization also fuels brand protection strategies because you can leverage each digital asset for consumer engagement to build confidence and trust. As we said in Part I of our consumer engagement series, your customers are absolutely a part of brand protection, and serialization empowers them in three important ways.
First, it’s the basis of an indelible, demonstrable, shareable product provenance that proves that your product is what you say it is and gives consumers the information they demand.
Second, as we discussed in Part II of our consumer engagement series, your serialized product is a device for one-on-one communication with your customers. For example, with a quick smartphone scan of a 2D Data Matrix code, a person can access rich data about your product and exclusive content through which they’ll establish and build a relationship with your brand.
Third, serialization lets you “crowdsource” brand protection through your consumer engagement activities. For example, if a person scans a product and the scan doesn’t work or they’re taken to a suspect website or other suspicious content, you can incentivize them to contact you and report that something is wrong. This is actionable, granular data that will protect your brand.
Real-time monitoring
A blind spot in your supply chain creates opportunities for trouble. Common blind spots include deviations from prescribed routes and harmful environmental conditions. The solution is real-time monitoring.
rfxcel’s Integrated Monitoring solution paints a vibrant, detailed picture of where your products are and what is happening to them. It provides better data, continuity, visibility, and security to protect your products and consumers. With detailed, unit-level data coming in around the clock, you’ll understand and immediately act upon specific risks.
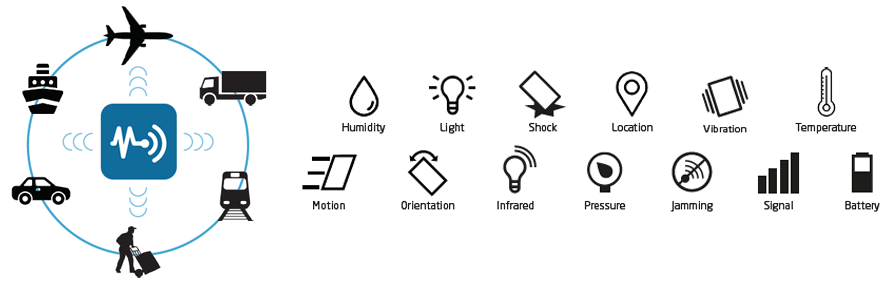
It works like this: Pallets, cases, or unit-level items are equipped with Internet of Things (IoT)-enabled sensors that send data over communication networks at regular intervals. The sensors provide real-time information about how long an item has been in transit, if the transport vehicle is sticking to its approved route, if the shipment stopped and for how long, and environmental conditions such as temperature, light, humidity, and shock. If something is not as it’s supposed to be, the sensors transmit an alert so you can halt shipments that may have been adulterated and redirect shipments to keep products safe. You’ll protect your customers and safeguard your brand.
Check out our short videos about our Integrated Monitoring solution for the pharmaceutical and food and beverage industries.
End-to-end traceability
End-to-end traceability in a digital supply chain means you can design end-to-end brand protection strategies.
With a suite of solutions acting in concert — like our Traceability System — you’ll create a full, traceable provenance for every product. You can add critical tracking events and key data elements at any point in your supply chain. For example, add a photo of a product as it leaves the factory or integrate a quality inspection to enrich the data associated with the product. Consumers can access this information and confirm that your product is what you say it is. This burnishes your reputation and builds trust with the people who buy your products or are thinking about buying your products.

And there are numerous other benefits of end-to-end traceability. If you can see every part of your supply chain from one end to the other, you’ll be able to manage operations more efficiently, including dealing with recalls and other crisis situations. You’ll make it harder for counterfeits and fakes to reach consumers. You’ll consolidate data to improve processes, outcomes, and product quality. And you’ll be empowered to make better decisions based on that data.
Mobile traceability: an on-the-go solution for brand protection strategies
A discussion of brand protection strategies isn’t complete without mentioning mobile traceability. Why? Because if you don’t have access to your supply chain data 24/7, no matter where you are, you’re not shedding light on blind spots and not building a solid perimeter around your brand.
rfxcel’s MobileTraceability solution brings our industry-leading track and trace capabilities to virtually any location, including places that traditionally may not have had absolute visibility in your supply chain, such as remote fields, distribution centers, and warehouses. With unparalleled convenience and speed, it gives you everything you need to keep you supply chain running at optimal efficiency. To learn more, read about what our Mobile Traceability solution can do for the pharmaceutical and food and beverage supply chains.
Final thoughts
A supply chain problem is a brand protection problem. By giving all your products a unique digital identity through serialization, then following and controlling them in real time with integrated monitoring and traceability solutions, you can demonstrate to the world that your brand is strong and reliable. You can build a reputation for integrity and transparency with partners and consumers. You can use data to innovate your brand protection strategies, consumer engagement activities, and every part of your operations.
With rfxcel, you can fortify your brand with data from a digital supply chain. You can combat the threats we’ve discussed no matter where you are and no matter where you supply chain goes. Contact us today to see how our solutions can work for better, stronger brand protection strategies.