Welcome to the rfxcel DSCSA Compliance Library. It’s a collection of our key DSCSA resources (as of September 2023) and is a convenient place to get information as the industry navigates the “extended stabilization period” until FDA enforcement begins on November 27, 2024.
We will, of course, continue writing about the DSCSA and providing the best supply chain solutions to ensure manufacturers, wholesalers, dispensers, repackagers, third-party logistics providers — all pharmaceutical stakeholders — meet the requirements and remain compliant forever.
So bookmark this page and our blog. You can also subscribe to our newsletter to make sure you don’t miss anything about the DSCSA and other important developments in the pharma industry. Just fill out the short form at the bottom of any page on our website.
And of course, contact us today with your questions about the DSCSA or anything else about the pharma supply chain. Our experts are here to help.
A note about the rfxcel DSCSA Compliance Library
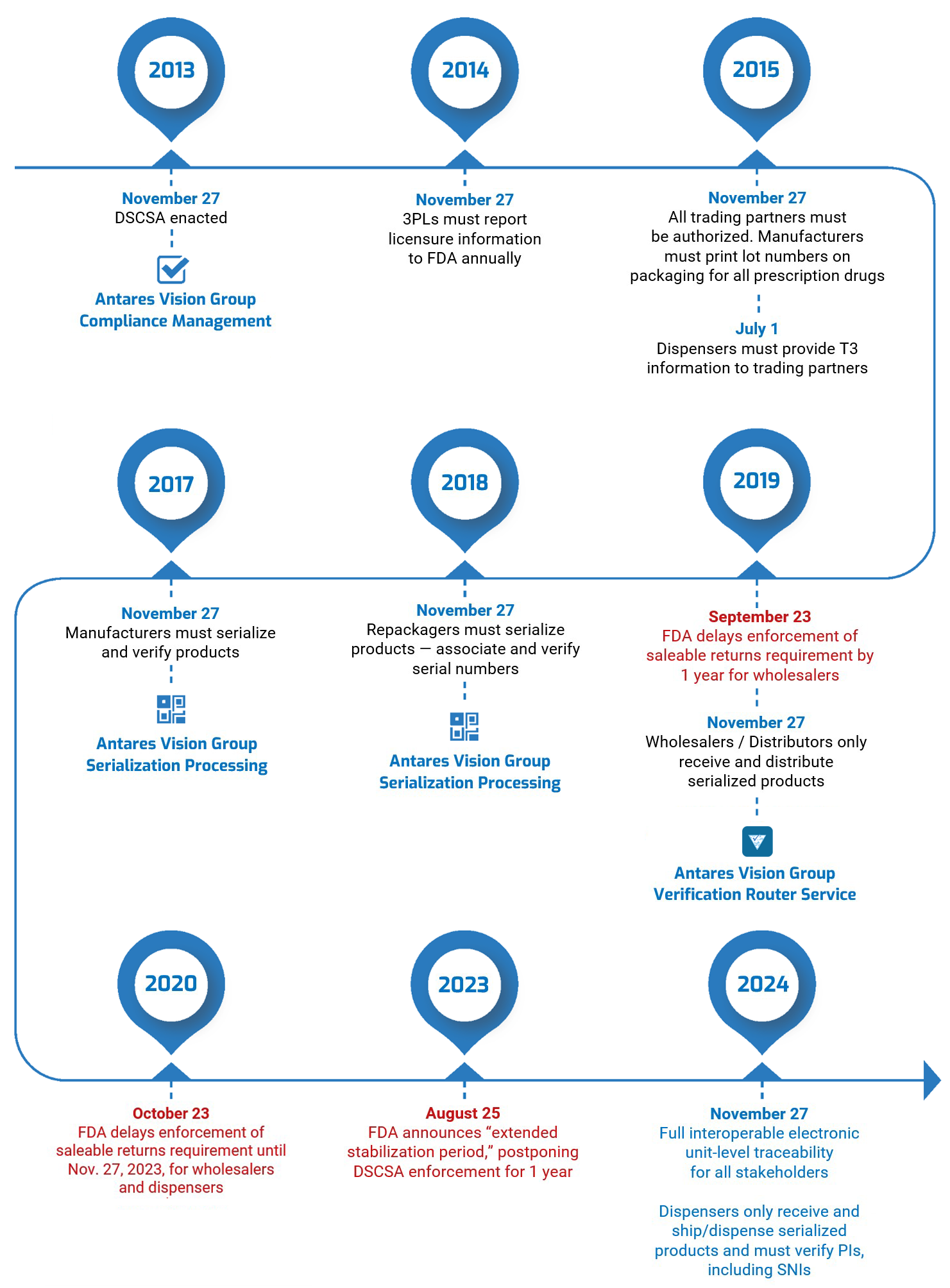
Please note that that we haven’t included everything we’ve written or presented about the DSCSA. Deadlines and requirements have changed since the law was enacted in November 2013, so some of our earlier pieces are, logically, outdated.
The DSCSA in our blog
- FDA Postpones Enforcement of Key DSCSA Requirements to November 27, 2024
- FDA National Drug Code: Proposed Format Changes & Industry Impact
- DSCSA Summary: A Look at the Law as We Count Down to 2023
- DSCSA Compliance Update: Herb Wong Tells Us What’s Happening Now
- FDA DSCSA Guidance Update: EPICS, ATPs, and the Countdown to 2023
- What Is the Drug Supply Chain Security Act?
- Drug Supply Chain Security Act Pharmacy Responsibilities
- What Are the Two Parts of the Drug Quality and Security Act (DQSA)?
- Countdown to DSCSA 2023 Serialization: The Deadline Is Two Years Away
- FDA Official Says DSCSA 2023 Interoperability Deadline Will Not Change
- DSCSA 2023: Understanding DSCSA Authorized Trading Partners, Part 1
- DSCSA 2023: Understanding DSCSA Authorized Trading Partners, Part 2
- DSCSA ATPs: Top Authorized Trading Partner Questions, Answered
- DSCSA 2023 and EPCIS: Your Top Questions, Answered
- DSCSA VRS: Top Verification Router Service Questions, Answered
- DSCSA 2023: The Future of Pharmaceutical Traceability in the United States
- DSCSA Saleable Returns Verification Requirement: Just the Facts
- FDA Delays Enforcement of DSCSA Saleable Returns Requirement
- Industry Reaction to Delayed Enforcement of DSCSA Saleable Returns
- rfxcel DSCSA 2023 Webinar Series: Sneak Peek #1
- rfxcel DSCSA 2023 Webinar Series: Sneak Peek #2
- rfxcel DSCSA 2023 Webinar Series: Sneak Peek #3
DSCSA compliance white papers
- The DSCSA: Preparing for the Full Serialization of the U.S. Pharmaceutical Supply Chain
- DSCSA Verification Router Service Basics
Our top DSCSA news items
- Antares Vision Group Becomes First DSCSA Compliance Software to Be Awarded a Spot on the GSA MAS Contract with Lovell Government Services
- rfxcel Announces EPCIS Center of Excellence to Enable DSCSA Serialization Requirements by 2023
- rfxcel to Integrate Spherity Credentialing Service into Its VRS Solution for DSCSA Authorized Trading Partner Compliance
- rfxcel Adopts Open Credentialing Initiative (OCI) to meet DSCSA Authorized Trading Partner (ATP) requirements
- Ohio Department of Veterans Affairs to Utilize rfxcel’s Drug Supply Chain Security Act Compliance Software
- rfxcel Begins Verification Router Service Pilot for the FDA
Our top DSCSA webinars
- Plan for DSCSA Readiness
- DSCSA 2023 webinar series (all three in one place):
- The Verification Router Service: Aligning to the Standard
- ASN to EPCIS: Industry Change, Your Challenge
- Authorized Trading Partners: The OCI Solution
The DSCSA timeline