Full serialization of the U.S. pharmaceutical supply chain is coming on November 27, 2023. To help make sure you have the latest information and are doing everything you can to be prepared, we’re hosting the rfxcel DSCSA 2023 webinar series on June 15, 16, and 17.
Our Executive Global Advisor Brian Files, an expert on U.S. and international pharmaceutical compliance, will present three key aspects of the DSCSA and answer your questions. Sign up today!
- Tuesday, June 15: The Verification Router Service: Aligning to the Standard
- Wednesday, June 16: ASN to EPCIS: Industry Change, Your Challenge
- Thursday, June 17: Authorized Trading Partners: The OCI Solution
Here’s a sneak peek about DSCSA authorized trading partners (ATPs). Check back for one last sneak peek before Brian kicks off the webinars on June 15!
What is the DSCSA?
The DSCSA went into effect on November 27, 2013. In addition to ATPs, it calls for product tracing, product identifiers (PIs), and verification requirements for manufacturers, wholesale distributors, repackagers, and dispensers (pharmacies). As we said above, full serialization is scheduled to begin on November 27, 2023.
What are ATPs?
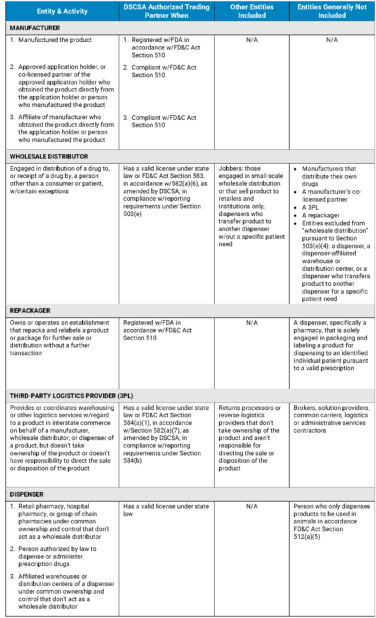
Under the DSCSA, manufacturers, wholesale distributors, repackagers, third-party logistics providers (3PLs), and dispensers are ATPs. They may engage in transactions only with other ATPs.
In other words, these supply chain actors and their trading partners must be ATPs. (In broad terms, a trading partner is an entity that accepts or transfers direct ownership of a product from or to a manufacturer, repackager, wholesale distributor, or dispenser.) If you are not an ATP, your access to the U.S. pharma supply chain will be severely restricted or denied altogether.
ATP requirements at a glance
To be considered an ATP, manufacturers, wholesale distributors, repackagers, 3PLs, and dispensers must meet the criteria presented in the table below, which we’ve adapted from an August 2017 FDA publication.
Final thoughts
Be sure to join us on June 15, 16, and 17 for our DSCSA 2023 webinar series. Register today and submit your questions for Brian. You can also contact us to talk with one of our supply chain experts and see how our award-winning rfxcel Traceability System can ensure you comply with all DSCSA requirements.
See you on June 15!






