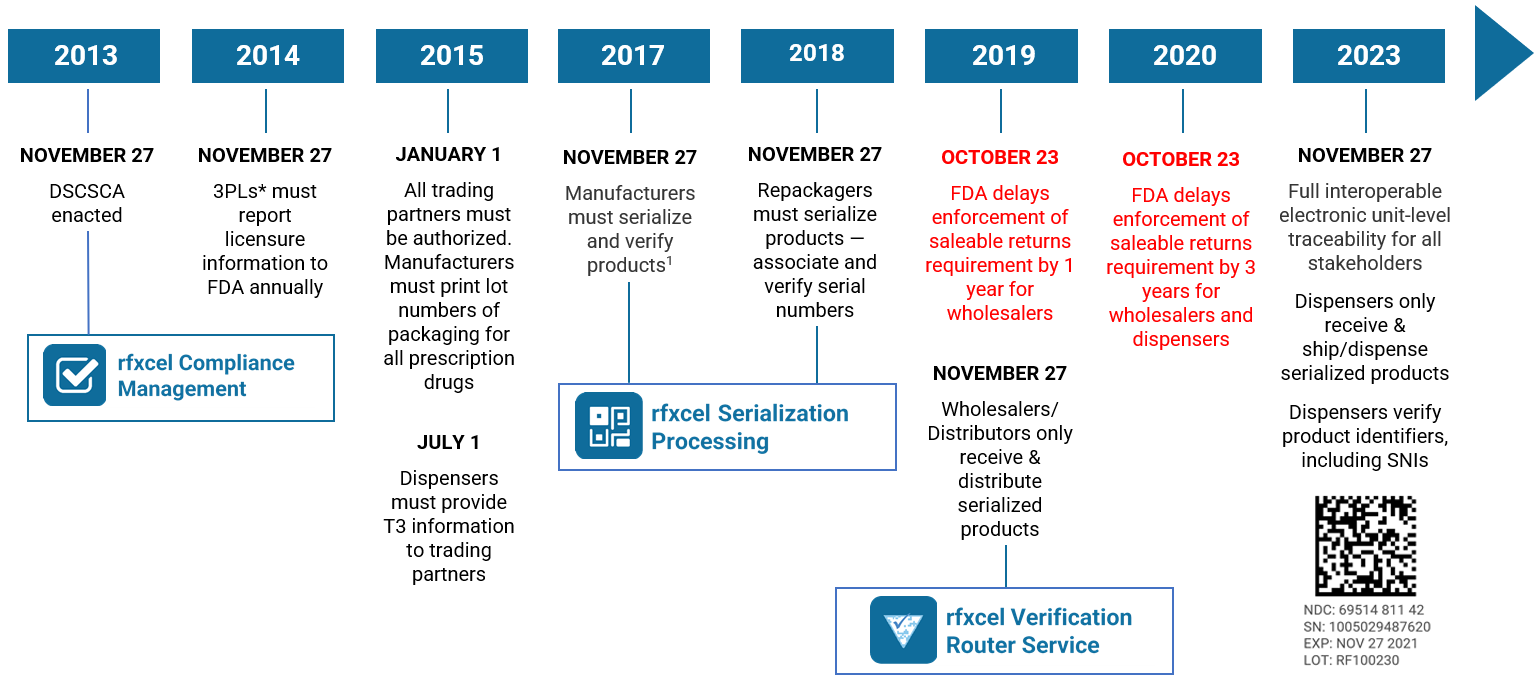
We just did two articles about why your supply chain is vital to an effective consumer engagement strategy. Now we want to jump into brand protection strategy and your supply chain.
First, let’s define our terms. Although consumer engagement is a usually a dedicated effort to boost brand recognition and loyalty, it must always be considered part of your brand protection strategy. As we’ll see, getting your customers involved in fighting counterfeits and identifying disreputable sellers and other bad actors is critical. Let’s continue breaking this down.
Why do you need a brand protection strategy?
Do you want to protect your business? Your employees, your bottom line, your reputation, your supply chain, your intellectual property?
Of course you do. Your brand protection strategy is your firewall. It’s how you shield your business from things that can harm it. And many — if not most — of the factors that can harm your brand are directly related to your supply chain. These include counterfeits (also called fakes), diversion, theft, and insufficient traceability.
Top supply chain threats
Let’s take a closer look at the top supply threats your brand protection strategy should address.
Counterfeits and fakes
The joke is that the best way to fight counterfeits is to make products nobody wants to buy.
We know that’s not how it works, though, which is why counterfeits and fakes are the No. 1 brand protection concern. In fact, counterfeits and pirated products accounted for up to 3.3 percent of world trade in 2016.
That statistic comes from a 2019 report by the Organisation for Economic Co-operation and Development (OECD) entitled “Trends in Trade in Counterfeit and Pirated Goods.” The OECD also found that trade in counterfeit and pirated goods was rising steadily despite stagnation in overall trade volumes. Based on 2016 customs seizure data, the value of imported fake goods worldwide was $509 billion, up from $461 billion in 2013.
But there’s a lot more to be concerned about. Counterfeits are of inferior quality and often contain harmful, even deadly, materials/ingredients. The people who make them, including children, often work for long hours in sweatshop conditions. Some may have been trafficked or coerced. Furthermore, it has been established that counterfeits are inextricably linked to organized crime.
The pandemic provided many examples counterfeits making their way into the global supply chain — fake vaccines, fake COVID-19 testing kits, fake masks, fake nitrile gloves. But counterfeiting affects every industry, from food and footwear to cosmetics and computers.
Diversion and theft
When your goods are in transit along your supply chain, you want them to reach their final destination as quickly and safely as possible. This is why diversion is another key consideration for a brand protection strategy.
Diversion is actually a two-pronged problem. Let’s use pharmaceuticals to illustrate. Many drugs must be kept within a certain temperature range or maintained under certain lighting or humidity conditions. Even the slightest delay could spell disaster — ruined products, which means patients might not get medicines on time.
Diversion can also indicate theft. If a truck goes off its prescribed route, bad actors might be hijacking it and your product could end up on unauthorized e-commerce sites (rogue websites) and other grey markets or black markets. If the diversion has compromised the integrity of your product — a drug, for example — people’s lives may be jeopardized.
Insufficient traceability
As we’ve discussed before, supply chain traceability brings tangible value to just about every part of your business, including your brand protection strategy. If you’re not taking traceability seriously, you’re not just opening the door to assaults on your brand; you’re risking problems with regulators, alienating (and losing) customers, and weakening your supply chain.
A recall is among the most damaging events that can happen to a brand, so let’s use it as case study. If you can trace a recalled item, you can better collaborate with trading partners and authorities and help to get the product out of the supply chain and out of stores. With traceability, you’re protecting consumers from a health hazard and safeguarding your brand from bad publicity. And with a transparent approach to engaging with customers about your products, you create a strong brand image that conveys trust, credibility, and reliability.
Traceability also helps fight counterfeits, diversion, and theft. The ability to trace and authenticate every product in your supply chain in real time, 24/7, is foundational to an effective brand protection strategy. We’ll get into those details in Part II of our brand protection series.
Final thoughts
At the end of August, the Office of the United States Trade Representative published a request for comments “that identify online and physical markets to be considered for inclusion in the 2021 Review of Notorious Markets for Counterfeiting and Piracy (Notorious Markets List).”
Counterfeits have also been making headlines in recent weeks:
- “Counterfeit products flood internet ahead of holidays”
- “Watch out for counterfeit items when shopping online this holiday season”
- “Toy Association Warns Holiday Shoppers about Counterfeit Toys Sold Online”
In this environment, a comprehensive brand protection strategy driven by granular supply chain data is your best defense against bad actors. rfxcel understands this. We can help you leverage your supply chain to combat counterfeits and the other concerns we addressed today. Our brand protection solutions will fortify your brand with data from a digital supply chain. Contact us today to learn more — and read Part II of our brand protection series.