The Food Safety Modernization Act (FSMA) is transforming the food supply chain in the United States. Learn about FSMA requirements and how they affect the food industry.
Understanding the FDA Food Safety Modernization Act (FSMA)
The Food Safety Modernization Act (FSMA), signed into law in 2011, aims to reduce foodborne illness, protect the U.S. food supply, and ensure public health. The law gives the U.S. Food and Drug Administration (FDA) authority to regulate the production, processing, packing, and transport of food throughout the country.
The FDA has finalized nine major rules through FSMA that address different aspects of the food supply chain. FSMA covers both human and animal food, and the rules are designed to address issues such as traceability, sanitation, produce safety, and supplier verification.
7 FSMA rules and requirements
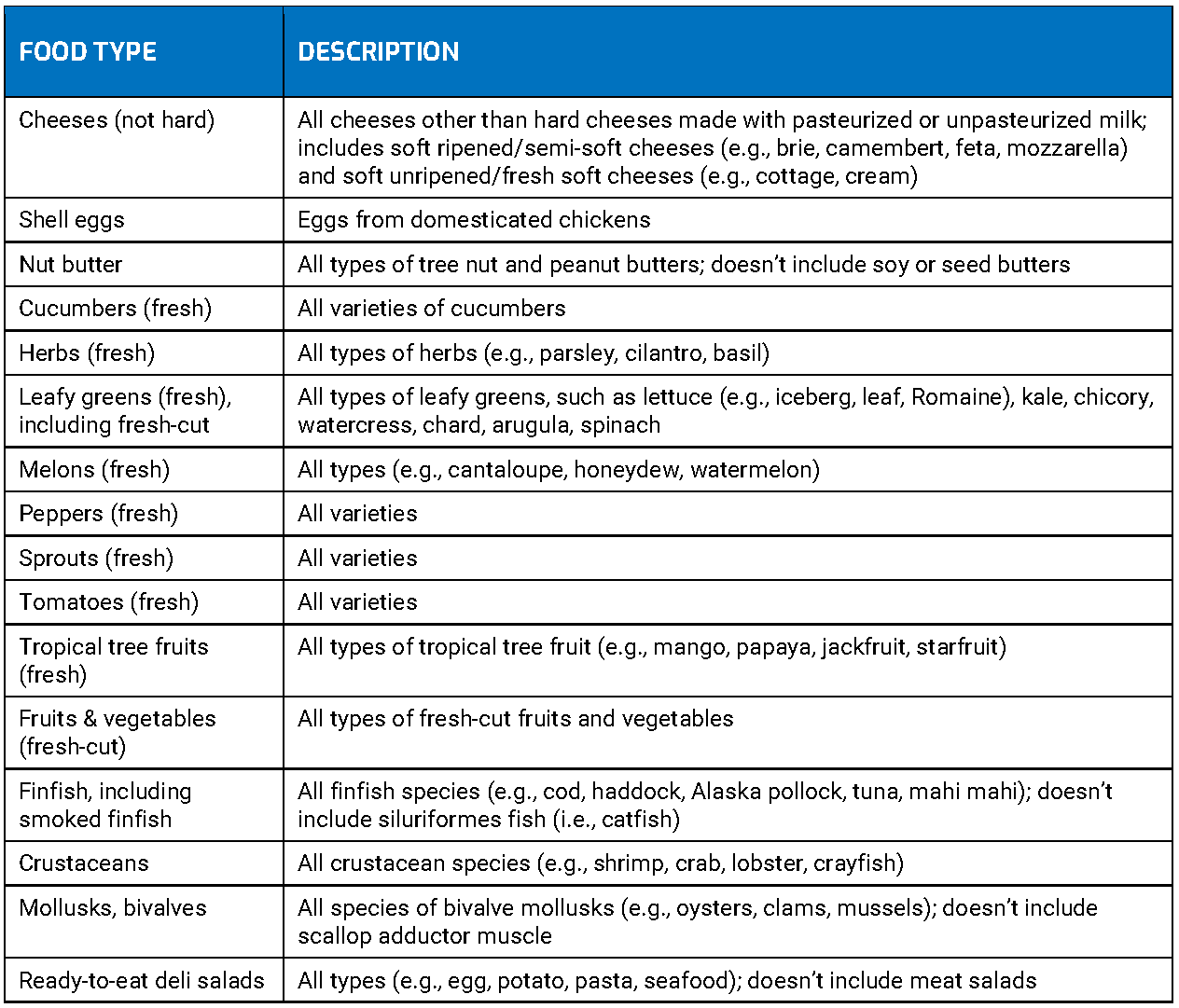
Through FSMA, the FDA has issued rules that govern food production and distribution. The food traceability final rule established additional traceability recordkeeping requirements for persons who manufacture, process, pack, or hold foods included on the Food Traceability List (FTL). Additionally, there are seven main roles that the FDA implemented in the final FSMA rules.
-
-
- Preventive Controls for Human and Animal Food: Require food facilities to meet Current Good Manufacturing Practice (CGMP) requirements, conduct hazard analyses, and establish risk-based preventive controls.
- Produce Safety Rule: Established science-based standards for the production, packing, and storage of fruits and vegetables on farms in the U.S. and other countries.
- Foreign Supplier Verification Program (FSVP): Requires foreign suppliers to show that they are meeting food safety standards required in the U.S.
- Sanitary Transportation Rule: Established regulations for the sanitary transportation of human and animal food.
- Accredited Third-Party Certification Rule: Created a program to accredit specific third-party certification bodies to conduct food safety system audits of foreign facilities.
- Protection Against Intentional Adulteration: Aims to address the probability of an outbreak due to potential food safety risks of intentional adulteration.
- Voluntary Qualified Importer Program (VQIP): fee-based program provided by the FDA FSMA to foreign food facilities that intend to import their products into the country.
-
Other rules concern protection against intentional adulteration of food and guidelines for the use of agricultural water.
Compliance strategies for FSMA
Navigating FSMA can be complicated, especially as rules are proposed, reviewed, and finalized. To implement effective compliance strategies, organizations should:
-
-
- Review: Start by conducting a thorough review of FSMA requirements to understand which rules apply to your business.
- Develop: Create a robust FSMA food safety plan that’s tailored to your operations, including hazard analysis and critical control points (HACCP).
- Implement: Establish preventive controls and monitoring systems across the organization and have a plan in place for food recalls.
- Verify: Use audits and validation procedures to ensure compliance.
-
Implementing FSMA’s Preventive Controls and Hazard Analysis
FSMA’s Preventive Controls for Human Food, finalized in 2015, was created to minimize and prevent hazards at food facilities. Key components of this rule include:
-
-
- Current Good Manufacturing Practice (CGMP) revisions: FSMA updated FDA’s existing CGMP requirements to create binding requirements for employee education and training.
- Food safety plans: The rule created new requirements for risk-based preventive controls and hazard analysis at food facilities, including sanitation practices and allergen controls.
-
Businesses covered under the Preventive Controls rule must perform a hazard analysis of their facilities and products, implement food safety practices, and provide ongoing monitoring and verification of all preventive controls.
Ensuring FSMA compliance in supply chain management
Even if you’re confident about your organization’s food safety practices, you need to know that your supply chain partners are holding themselves to the same standards. Establishing clear supplier approval and verification processes can help you thoroughly vet the growers, suppliers, processors, or distributors you work with.
By implementing traceability and documentation requirements, you’ll be able to track specific products and lots in real-time. As with your internal processes, conduct regular assessments to ensure suppliers and co-manufacturers are maintaining FSMA compliance.
Final thoughts: FSMA rules and regulations
The worldwide food industry should monitor events in the United States as FSMA traceability requirements evolve. It’s not just about compliance and being able to sell products in America; it’s about being able to anticipate regulatory trends, keeping your supply chain moving at peak performance, and leading in the industry through adaptation and innovation. It’s also about leveraging the FSMA regulations to create business opportunities.
We understand the importance of complying with FSMA and other regulations for the food and beverage industry. We have extensive experience delivering tailored traceability, visibility, and transparency solutions that not only help ensure compliance, but also create added value for operational efficiency, brand protection, and customer loyalty. Contact us today and one of our traceability experts will show you how it works.
And be sure to download our “Traceability in the Food Supply Chain” white paper, which explains the FSMA Food Traceability List and the Food Traceability Final Rule in detail.