Imagine for a minute that you get the phone call all companies dread. It’s the “I am dissatisfied with my product” call, direct from a customer. You take the call, help the customer, and move on, right? You’re feel good about solving the problem — and are definitely not thinking about supply chain traceability.
A few days go by, then you’re flooded with more complaints, none of which seem to be related. You scramble to identify the root cause of all your problems, but you don’t know where to look. What should you do?
This is when you should be thinking about supply chain traceability. It is the key to having total insight into how your products get made, where they’ve been, and where they’re going. By employing supply chain traceability, you can:
- Assure your brand and customers are protected
- Maintain regulatory compliance
- Pinpoint common problems, such as counterfeits and diversion
Let’s take a deeper look into each of these benefits.
1. Supply chain traceability for brand protection and customer satisfaction
Customers grade a brand on both the quality of its products and how it responds when an issue arises. Brands build loyalty with their customers by being transparent and demonstrating consistent product and service quality. Brands lose customers by failing to respond to complaints and recalls.
Response to complaints and recalls
Supply chain traceability is helpful when investigating a customer complaint. It is also essential when assessing a potential recall or managing an actual recall.
All the products you make and sell must have identification that traces back their source. Manufacturers and retailers can follow a problematic unit backward through its life cycle. Information collected through traceability includes:
- Date and time of manufacturing
- Equipment used for manufacturing and packaging
- Personnel involved in manufacturing and packaging
- Raw materials and components
- Physical locations of inventory throughout its life
In a best-case scenario, the complaint is an isolated incident that’s easy to fix. But if the complaint means there’s been a breakdown somewhere or a product has been compromised, a recall may be triggered.
Recalls happen for any number of reasons. For example, you might discover a material that is out of specification. Once identified, it is necessary to trace all usage of that material. Another example is contamination, especially in the food supply chain.
A recall of any size can have a significant impact on your business. Having a supply chain traceability system will speed recalls, improve cooperation with regulators and other authorities, enable better information-sharing with your customers, and reduce damage to your brand reputation. Faster batch recalls are possible only when there is a robust supply chain traceability system.
Transparency about sourcing
A study conducted by the Consumer Goods Forum found that “70 percent of consumers are most interested in transparency about products.” Customers want to know where the materials used in their products came from and how they were sourced.
Some companies are publishing reports to make their supply chains and operations more transparent — and to make their brand more appealing to consumers. In addition to how they source materials, they’re sharing information about things such as:
- Origins and purposes of raw ingredients
- Manufacturing processes
- Safe handling practices along the supply chain
- The brand’s mission and values
This type of “transparency marketing” is effective in the food and consumer packaged goods industries, as it entices consumers to make a purchase because they’re getting the product information they demand.
A 2016 study by Label Insight indicated that 94 percent of consumers are not only likely to be loyal to a brand that offers complete transparency but are willing to pay more for products that meet such standards. This has powerful implications for brands; it shows that transparency made possible with supply chain traceability inspires product and brand loyalty.
2. Supply chain traceability is key to compliance
Being able to track material movement and consumption is critical for regulatory compliance.
The ISO 9000 Standards Series is the basis for most industries’ standards and provides expectations to help companies structure their quality management systems. Within these standards, traceability is defined as “The ability to trace the history, application, use and location of an item or its characteristics through recorded identification data.”
To meet this standard, it’s critical to be able to identify individual product units. Also, you must collect information about subcomponents. This information will allow for the tracing of parts of products throughout your supply chain.
Serialization makes supply chain track and trace easier
Serialization is the process of assigning unique identifiers to outbound and inbound materials. This makes the parts of products easier to track and trace throughout your processes. Utilizing software such as our Serialization Processing solution will save you time and effort in managing your supply chain.
Collecting supply chain data on your materials and finished products can also help to identify problems before they become an issue. This empowers you to be proactive and assess what works well (or what does not work well) throughout your entire supply chain process, which allows you to standardize your work processes and cut out waste.
Prove your product claims
Marketing claims must be substantiated, not only to consumers but also to regulatory agencies. Claims about sustainable sourcing, organic certifications, and other attributes can be demonstrated with supply chain traceability. Safe and compliant handling of products is also best demonstrated with traceability. Many retailers and manufacturers have routine audits to assure that they have processes to trace a product’s life cycle. In some industries, the results of these audits are available as public information.
Regulations evolve over time
Regulatory requirements for the documentation of traceability are constantly evolving. For example, the U.S. Food and Drug Administration (FDA) published the “Proposed Rule for Food Traceability,” which called for additional traceability records for certain foods. The FDA is encouraging the voluntary adoption of these new practices for all food products. These new requirements will affect all who manufacture, process, pack, or hold foods.
We’ve written extensively about the FDA’s push for traceability in modern food supply chain. For example, check out “Food Traceability Regulations in the United States: A Timeline.”
rfxcel is prepared to help you comply with all current and future requirements in any industry, including food and beverage, life sciences/pharmaceuticals, government, and consumer goods. We track industry regulations and guidance documents for upcoming and proposed legislation. We have software solutions that take the guesswork out of compliance no matter where you do business.
3. Combat counterfeits and diversion/theft by identifying supply chain weak spots
One of the worst things that can happen to any business is having their products counterfeited or stolen.
Counterfeits not only result in a direct loss of sales, but your customers might lose faith in your brand. If a person winds up with an inferior counterfeit product with your name and logo on it, there’s a good chance they will be dissatisfied with your brand and take their business somewhere else. And if your products are diverted or stolen, then you’re losing money and might have a much larger supply chain problem on your hands.
Supply chain traceability is like a forensic tool to help fight counterfeits and diversion — and host of other problems. Here’s how it works:
- You know the origin of your ingredients. You can verify that all ingredients or components are legitimate. You can see the history of any ingredient, including its origin and when it was combined with other ingredients to make a finished product.
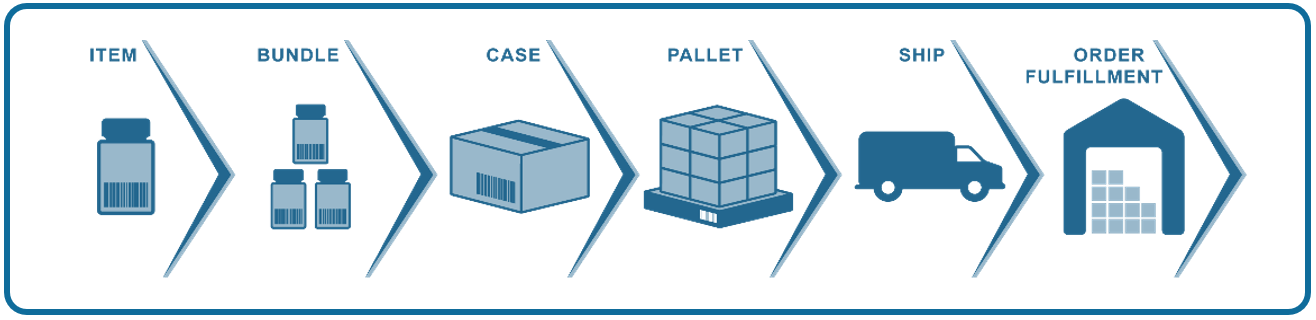
- You can trace a product’s every move. Traceability means you can see everywhere a product has been before, during, and after it was harvested or manufactured. You can see every case or box, every pallet, every delivery vehicle, every stop along the supply chain (e.g., a warehouse, a retail store, a pharmacy or hospital). Even after a product has been unloaded from the delivery vehicle and taken out of cases or boxes, you see where individual units have been right up to the time the consumer takes possession (check-out at the cash register, dispensation at a clinic, etc.).
- You can pinpoint where a product might have been harmed or compromised. Traceability data will show if a shipment strayed from its prescribed route, which could indicate theft or other mischief that could harm your bottom line and brand. Serialization, compliance, real-time monitoring, and other supply chain traceability solutions create a provenance that can demonstrate the legitimacy and purity of every product.
Final thoughts
We hope you have a better idea of how supply chain traceability can help your brand and business. Traceability is a crucial aspect of managing your business operations.
Are you ready to get started? Setting up your own supply chain traceability system might seem daunting, but rfxcel is here to help. We have easy-to-use, scalable solutions for all of your track and trace needs, no matter what industry you’re in.
Contact us today if you would like to see a short demo of how we can help you to build an effective traceability system. Together, we can protect your brand, ensure regulatory compliance, and fight counterfeits and theft.