Where would we be without standards? One thing’s certain: Your supply chain would be a lot messier, if not chaotic if you and your partners didn’t have a “common language” to process and exchange data. Standardized GS1 barcodes are one way we avoid this chaos.
GS1 barcodes keep everybody “on the same page.” They allow companies in virtually every industry to easily record, store, and transfer data. We’ve all seen them, and chances are your organization uses them — maybe even millions or billions of them. But let’s do a quick refresher course as another way to keep us all on the same page.
What is GS1, and why does everyone use GS1 barcodes?
GS1 is an international not-for-profit organization that develops and maintains a broad set of global standards used by businesses the world over. Of the various standards created by the organization, the GS1 barcode is undoubtedly the most well-known. Everyone from Amazon to eBay follows GS1 barcode standards, as these regulations help them easily share additional information about products with consumers and retailers.
A group of U.S. grocery retailers founded GS1 in 1973. Simultaneously, they created the first barcode labels. Since its inception roughly 50 years ago, GS1 has established itself as the leading authority on product identification regulations.
While GS1 general specifications have evolved over the years, the mission behind GS1 has remained unchanged. The organization is committed to promoting operational efficiency and supporting the sharing of information. It does so by providing e-commerce businesses, distributors, manufacturers, and retailers with an easy-to-follow set of labeling standards.
In 2023, GS1 barcodes are contributing to the proliferation of the global commerce ecosystem. Standardized produce labels like barcodes transcend borders, language barriers, and currencies. They enable members of the supply chain to interact on a worldwide scale to the benefit of consumers everywhere.
Why are GS1 barcodes important?
The short answer is, as we said above, standardized GS1 barcodes allow us to maintain order and avoid chaos. Workflows become quicker and more efficient. The GS1 barcodes keep supply chains running by enabling companies to sell, ship, track, reorder, and return products, in most cases by scanning with a handheld device or a camera-based system.
GS1 barcodes also expedite communication, traceability, visibility, and transparency. It’s really all about sharing information quickly in order to know the source of ingredients/components and products, where they’ve been and where they’re going, and when they reach their final destination.
These capabilities not only make supply chains more efficient — they also increase product safety and protect consumers. If there’s a recall, for example, a company can locate its products quickly, make sure shipments are stopped, remove items from stores, and share data with regulators and even consumers.
GS1 barcodes also save money. Administrative costs come down when everybody uses the same standards and has the same expectations. And because GS1 barcodes facilitate digital supply chains, they increase speed and reduce paperwork.
The bottom line is that GS1 barcodes provide members of the supply chain with easy access to product data. In turn, distributors, carriers, and retailers use barcode graphics to trace products throughout the supply chain, optimize operational efficiency, and ensure that consumers are receiving safe and authentic products.
GSI Identification Keys
GS1 standards define a set of unique identification codes, known as identification keys. GS1 says its identification keys “refer unambiguously to a real-world entity,” such as a product, a logistics unit, a physical location, a document, a service relationship, or another entity.
In other words, the ID keys let you quickly and conveniently access information about items in your supply chain and share it with your partners. Only GS1 members can build ID keys, which must include a GS1 company prefix. There are 12 ID keys:
- Global Trade Item Number (GTIN): identifies products and services, such as food and clothing
- Global Location Number (GLN): identifies parties and locations, such as companies, warehouses, factories, and stores
- Serial Shipping Container Code (SSCC): identifies logistics units, such as parcels and palletized products
- Global Returnable Asset Identifier (GRAI): identifies returnable assets
- Global Individual Asset Identifier (GIAI): identifies assets, such as equipment used in manufacturing and transportation
- Global Service Relation Number (GSRN): identifies relationships between service providers and recipients, such as hospital staff and members of brand “loyalty” or rewards programs
- Global Document Type Identifier (GDTI): identifies documents, such as shipping paperwork
- Global Identification Number for Consignment (GINC): identifies consignments, such as logistics units being transported in a container on a ship or airplane
- Global Shipment Identification Number (GSIN): identifies shipments
- Global Coupon Number (GCN): identifies coupons
- Component/Part Identifier (CPID): identifies components and parts
- Global Model Number (GMN): identifies a product’s model number
The GS1 standards also encompass data capture, including definitions of barcode and radio-frequency identification (RFID) data carriers, that allow ID keys and other data to be affixed directly to an object. Data standards also address the hardware to read and produce barcodes (e.g., scanners and printers), and hardware and software to connect the barcodes and RFID tags to business applications.
The different types of GS1 barcodes
All GS1 barcodes are “containers” that can hold different amounts information, such as serial numbers, batch numbers, GTINs, and expiration dates. As the image below from GS1 shows, there are four types, or “families,” of barcodes: EAN/UPC, two-dimensional (2D), DataBar, and one-dimensional (1D).
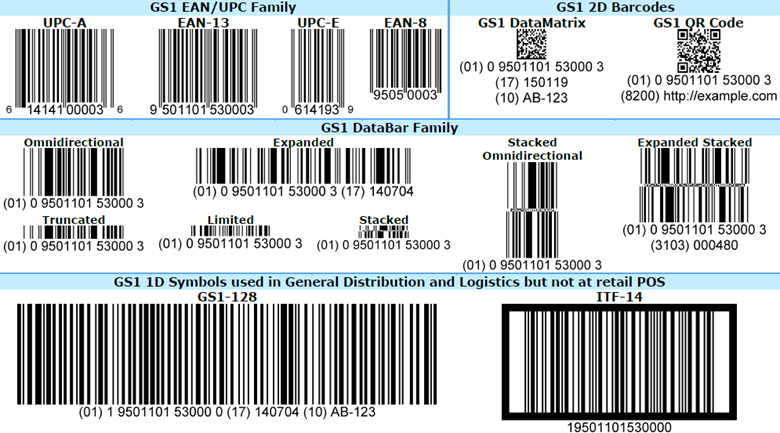
EAN/UPC family. GS1 says, rightly, that these “are printed on virtually every consumer product in the world.” They are characterized by a series of vertical lines and a horizontal row of numbers, and come in four types: UPC-A, EAN-13, UPC-E, and EAN-8.
These GS1 barcodes are designed to be used at the point of sale and can be read by omnidirectional scanners. None support attributes — they cannot contain product information such as an expiration date, a batch number, or item weight. UPC-A has 12 numbers and supports GTIN-12; EAN-13 has 13 numbers and supports GTIN-13; UPC-E has 12 numbers (the zeros are “suppressed) and supports GTIN-12; and EAN-8 has 8 numbers and supports GTIN-8.
2D barcodes. Like EAN/UPC barcodes, 2D barcodes are ubiquitous. They are incredibly robust, able to hold dense product information and remain legible at greatly reduced sizes or when they’re etched into a surface, such as a plastic bottle cap. There are two kinds of 2D barcodes:
- GS1 DataMatrix codes are omnidirectional and support attributes and all GS1 ID keys. They can hold 3,116 numeric or 2,335 alphanumeric characters.
- GS1 QR codes are also omnidirectional and support attributes and all GS1 ID keys. They can hold 7,089 numbers or 4,296 alphanumeric characters.
DataBar family. There are seven members in the DataBar family. Generally, they’re divided into two groups: those designed for use at the point of sale (i.e., can be read by omnidirectional scanners) and those that are not.
The first group has four types: omnidirectional, stacked omnidirectional, expanded, and expanded stacked.
- Omnidirectional and stacked omnidirectional have 14 numbers. They support GTINs and Global Coupon Numbers (GCNs) but do not support attributes.
- Expanded and expanded stacked have a maximum capacity of 74 numeric and 41 alphabetic characters. They support GTINs and GCNs, but do support attributes.
There are three types of barcodes in the second group: truncated, limited, and stacked. These have 14 numbers and support GTINs, but do not support attributes. They are not designed for use at the point of sale, so they cannot be read by omnidirectional scanners.
1D barcodes. The two types of 1D barcodes — GS1-128 and ITF-14 — are used in retail distribution, healthcare, and logistics. GS1-128 barcodes can carry any GS1 ID key and up to 48 alphanumeric characters, including serial numbers, expiration dates, and other information that helps track products through a supply chain. More than one GS1-128 barcode can be used on a single item. ITF-14 barcodes can hold only GTINs; GS1 says it is suitable for printing on corrugated materials.
Final thoughts
This year was the 50th anniversary of the GTIN. As GS1 said, “It is no exaggeration to say that the development of the GTIN set the stage for global, digitalized commerce.” Indeed, labeling standards and barcode technologies have evolved and advanced since 1971 to the great benefit of businesses and consumers alike.
We have been talking about the advantages of end-to-end traceability in a digital supply chain for a long time. When your products, labeled with powerful identifiers such as 2D DataMatrix codes, move through a digital supply chain powered by our award-winning Traceability System, you can leverage rich, unit-level data for much more than compliance and operational efficiency: You can create genuine, tangible business value. For example:
- You can develop better brand protection strategies, including fighting the top supply chain threats (e.g., counterfeits and theft).
- You can create next-level customer engagement programs and strategies.
- You can improve recall management.
- You can build your brand reputation and gain consumer trust.
That’s really just the beginning of what a digital supply chain can do. To learn more, contact us today to see a short demo of our solutions. Our supply chain experts will show you how our Traceability System transforms your supply into your most valuable strategic asset.

