The Drug Supply Chain Security Act (DSCSA) was passed 10 years ago in November 2013. Congress created the legislation to secure the U.S. pharmaceutical supply chain through unit-level product identification (serialization) and electronic exchange of product information.
Over the years, the FDA has issued updates and revised DSCSA guidance for manufacturers, dispensers, wholesale distributors, and other pharma stakeholders. If your products and/or operations are regulated by the law, it is vital to remain aware of requirements, changes, and deadlines.
Let’s explore the DSCSA guidance and requirements and what the FDA has done in recent years.
What Is the DSCSA?
Created as Title II of the Drug Quality and Security Act (DQSA), passed by Congress in November 2013, the DSCSA is an initiative to prevent the introduction and distribution of counterfeit, stolen, contaminated, or otherwise harmful drugs in the United States. It outlines steps to build an interoperable electronic system to identify and trace prescription drugs as they are distributed throughout the country.
Who Must Comply with the DSCSA?
Manufacturers, wholesale distributors, repackagers, dispensers (i.e., pharmacies, healthcare systems), and third-party logistics providers (3PLs) all have requirements with which they must comply.
Recent DSCSA Guidance Updates
Recently, the most notable action from the FDA was its announcement in August 2023 that it was delaying by one year enforcement of key DSCSA requirements. This “extended stabilization period” moves the enforcement date to November 27, 2024.
This DSCSA guidance primarily affects manufacturers, wholesale distributors, dispensers, and repackagers; delayed enforcement pertains to product identifiers (PIs) at the package level; saleable returns; interoperable, electronic product tracing; and investigating suspect and illegitimate products.
Though this gives the industry more time to comply, the Agency has made it clear that the postponement doesn’t amount to a grace period. It said the stabilization period was “not intended to provide, and should not be viewed as providing, a justification for delaying efforts by trading partners to implement the enhanced drug distribution security requirements.”
You can read the FDA’s official document about the stabilization period here.
Other Noteworthy DSCSA Guidance Updates
July 25, 2022: The FDA published a proposed rule, “Revising the National Drug Code Format and Drug Label Barcode Requirements.” The National Drug Code, or NDC, is the Agency’s “standard for uniquely identifying drugs marketed in the United States.” The codes are usually found on product labeling and might be part of the universal product code (UPC). Read more about the NDC and what the FDA said here.
October 23, 2021: In a policy document, the FDA announced it was delaying enforcement of key requirements to verify saleable returns. It also included guidance for wholesale distributors concerning transaction statements under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
The timeline below provides an at-a-glance view of DSCSA guidance as the law has been rolled out over the last decade.
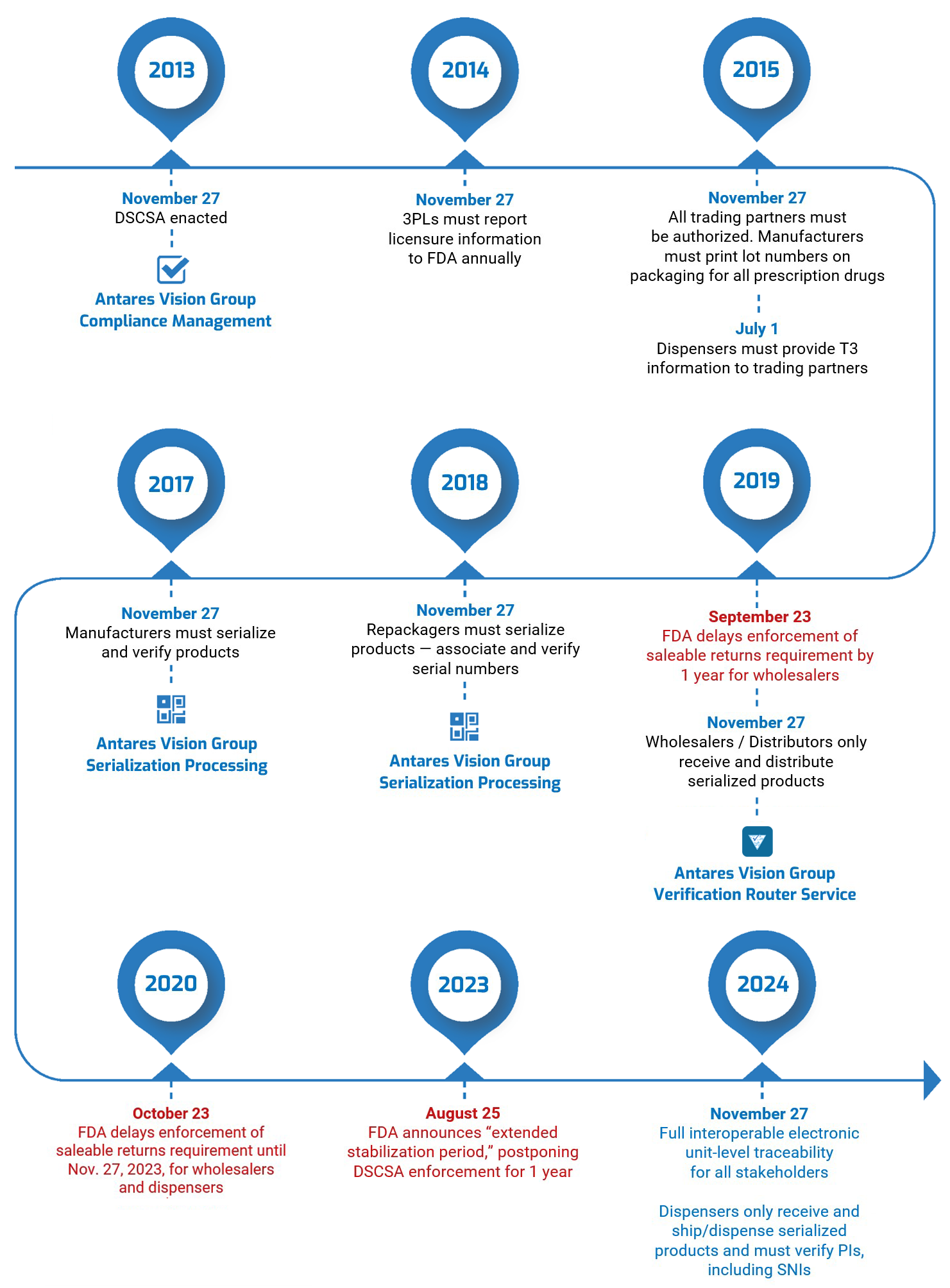
DSCSA Guidance in Context, Today
The stabilization period announced in August 2023 did not, in fact, change the original compliance deadline of November 27, 2023; it is up to individual states to decide if they’re going to enforce the requirements before November 2024.
However, the FDA said extending enforcement will give supply chain stakeholders the extra time that may be necessary “to continue to develop and refine appropriate systems and processes to conduct interoperable, electronic tracing at the package level, to achieve robust supply chain security under the DSCSA while helping ensure continued patient access to prescription drugs.”
What DSCSA Requirements Are in Effect Right Now?
It follows from what we just said that there is DSCSA guidance in effect right now. Some digressions are prohibited per the FD&C Act and can be enforced — with consequences ranging from product seizure to fines to imprisonment. Both federal and state authorities may take action against DSCSA violations.
What Was Enforceable Before Nov. 27, 2023?
Here are a few regulations that can be enforced now. For a full list, see the National Association of Boards of Pharmacy’s (NABP’s) excellent article here. (And also be sure to read about how we were the first DSCSA solution provider to join NABP’s Pulse Interoperable Partner Program. Learn more here!)
- All trading partners must be authorized trading partners (ATPs) and can only buy, sell, or trade with other ATPs.
- ATPs must be able to identify and manage suspect and illegitimate products.
- A product identifier (PI) must be placed on all regulated drug packages and homogenous cases — except for grandfathered products or products with an FDA waiver, exception, or exemption.
- ATPs must provide certain information about a drug and who handled it each time it’s sold: transaction information (TI), transaction statement (TS), and transaction history (TH).
What Went into Effect on Nov. 27, 2023?
The November 2023 deadline was when enhanced security requirements went into effect. We’ve written extensively about this for years (in our recently updated DSCSA white paper, for example), but here’s a summary of what companies must do to comply:
- Exchange TI and TS securely, electronically, and interoperably. TI must include each package’s unique identifier.
- Verify PIs at the package level.
- Respond to appropriate tracing requests and trace products at the package level (serialization).
- Associate saleable returns with the TI and TS associated with its initial sale.
Final Thoughts About DSCSA Guidance
So what’s the upshot of all this information? It’s simple: Don’t stop preparing.
Use your extra time during the stabilization period to evaluate your systems, communicate and coordinate with your trading partners, and — importantly — ensure you’re working with a solution provider that knows the DSCSA guidance inside and out.
If you have questions about the DSCSA or are concerned that your current provider may not have the tools you need to comply, we encourage you to contact us today to speak with one of our DSCSA experts. We are committed to meeting DSCSA compliance for all our customers in a timely manner.

