When do I need to implement the DSCSA VRS? Are there any new developments the industry should be aware of? What are some of the “sticking points” with the VRS today?
These were just a few of the questions asked during the first of three “DSCSA 2023” webinars that we held last month. In “The Verification Router Service: Aligning to the Standard,” rfxcel VP of Marketing and Strategic Initiatives Herb Wong and Global Executive Advisor Brian Files answered these and other questions about the DSCSA VRS. Below, we give their answers to the most-asked questions.
Check back throughout the week, because we’ll be posting the top questions from the other two webinars in the series, “ASN to EPCIS: Industry Change, Your Challenge” and “Authorized Trading Partners: The OCI Solution.”
The webinars were part of our ongoing efforts to keep pharma stakeholders up to date with the DSCSA and help the industry prepare for the full serialization of the U.S. pharma supply chain in November 2023. If you have other questions or want more details about DSCSA 2023, contact us today. You can also watch the webinars and download the presentation slides here.
When do I need to implement the DSCSA VRS?
Manufacturers and wholesalers/distributors should be implementing right now. VRS is a cornerstone of the DSCSA; it’s not going away. As you go through the 2020 to 2023 period, working with your partners is going to be critical. You should also be working with your solution provider — or finding one if you don’t already have one. Keep your eyes on the November 27, 2023, deadline and always be working toward it so you’ll be ready and compliant. Dispensers should be looking carefully at the benefits of VRS and requirements for validating saleable returns. (See response to next question.)
Are there any new developments the industry should be aware of?
VRS is the first interoperable system in the DSCSA. Error management and handling the complexities involved with the enormous volume of returned products contributed to its delay until 2023. (Read our articles about the FDA’s decision to delay enforcement of the DSCSA saleable returns requirement.) Downstream partners only add to the volume and complexity the VRS must handle in sub-second time. So, it will be important for the industry to determine exactly how the VRS will be used and what type of volume controls and error management it will have. You must also consider what type of outcomes your partners will need, as well as what you need for your own business model.
What are some of the “sticking points” with the VRS today?
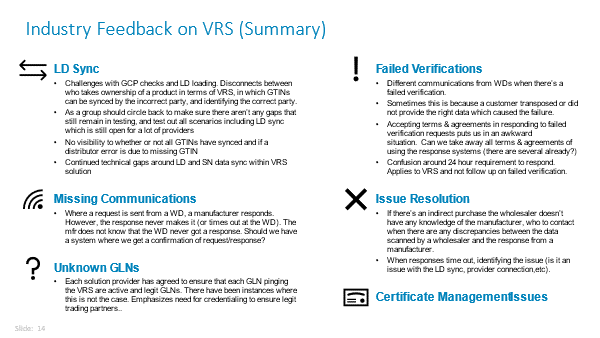
The Healthcare Distribution Alliance (HDA) collected feedback from the industry about optimizations/improvements it would like to see in the VRS network and presented its findings to solution providers on June 11, 2021. We’re now in the process of evaluating the feedback to determine next steps.
The “sticking points” fall into six categories, as shown in the graphic below. The most predominant concern is how to deal with data synchronization issues. The process for resolving all these issues needs to be streamlined among service providers.
What’s the current implementation rate and use of VRS?
That depends on which part of the supply chain you are referring to. There is no accurate estimate of this across the industry, but based on our observations, this is what we’re seeing: Manufacturers and distributors have the highest “implementation rate.” Approximately 70-80 percent of our manufacturers can support VRS and 80-90 precent of wholesalers/distributors are VRS ready. The numbers further down the supply chain are lower, but are increasing quickly as dispensers become more aware of the benefits of VRS.
My wholesale distributor takes care of VRS for me. What is my responsibility? Am I covered if I were to be audited?
This is a little tricky, because there’s a lot of information circulating about what wholesale distributors will and will not do in the VRS ecosystem.
Wholesale distributors are doing a lot of heavy lifting with VRS, but they’re not completely responsible for your DSCSA transactions. They’re responsible for your information that’s being plugged into the VRS, but they are not responsible if there are any problems with a returned product.
The simple truth is that every stakeholder is responsible for their own DSCSA compliance. Your wholesale distributor should be there to help coordinate to the extent of the arrangement and partnership you have, but they are not responsible for your compliance. It’s not their job to “take care of VRS” for you. As we get into 2023, you’re going to need hardware, software, and system updates ready to go, and you can’t “pass the buck” for VRS to your wholesale distributor — or any other trading partner.
More DSCSA 2023 resources from rfxcel
- DSCSA: November 2023 and the Full Serialization of the U.S. Pharma Supply Chain
- DSCSA 2023: Top EPCIS Questions, Answered
- DSCSA 2023: Understanding DSCSA Authorized Trading Partners, Part 1
- DSCSA 2023: Understanding DSCSA Authorized Trading Partners, Part 2
- DSCSA 2023: The Future of Pharmaceutical Traceability in the United States
- ATP Credentialing in the rfxcel VRS solution
- Plan for DSCSA Readiness webinar with Herb Wong (March 31, 2021)





