
India iVEDA Compliance
On April 1, 2020, India launched the Integrated Validation of Exports of Drugs from India and its Authentication (iVEDA) portal, officially replacing its Drugs Authentication and Verification Application (DAVA). iVEDA’s primary purpose is to be a repository database for serialized batch data, rather than means to focus on tracking and tracing the country’s drug supply. It was designed to help manufacturers and exporters use tertiary and secondary-level coded data more easily, effectively, and efficiently.
The iVEDA compliance deadline is February 1, 2024, for both small-scale industry (SSI) and non-SSI drugs.
India is a huge market with a complex regulatory landscape — and we can help you navigate it.
iVEDA requirements
Using the iVEDA portal, manufacturers and exporters must upload barcode data from secondary and tertiary packaging of drugs meant for export. A manufacturer that uploads this data must provide the manufacturer code and product code provided by GS1 India. They can choose to maintain the parent-child relationship between secondary and tertiary packaging, but it’s not mandatory. Additionally, manufacturers must have a Class-II or Class-III Digital Signature Certificate issued by any Certifying Authority in India.
Manufacturers are responsible for the accuracy, timeliness, and completeness of the data uploaded to iVEDA. They can, however, extend this responsibility to wholesalers, distributors, retailers, or anyone next to them in the supply chain.
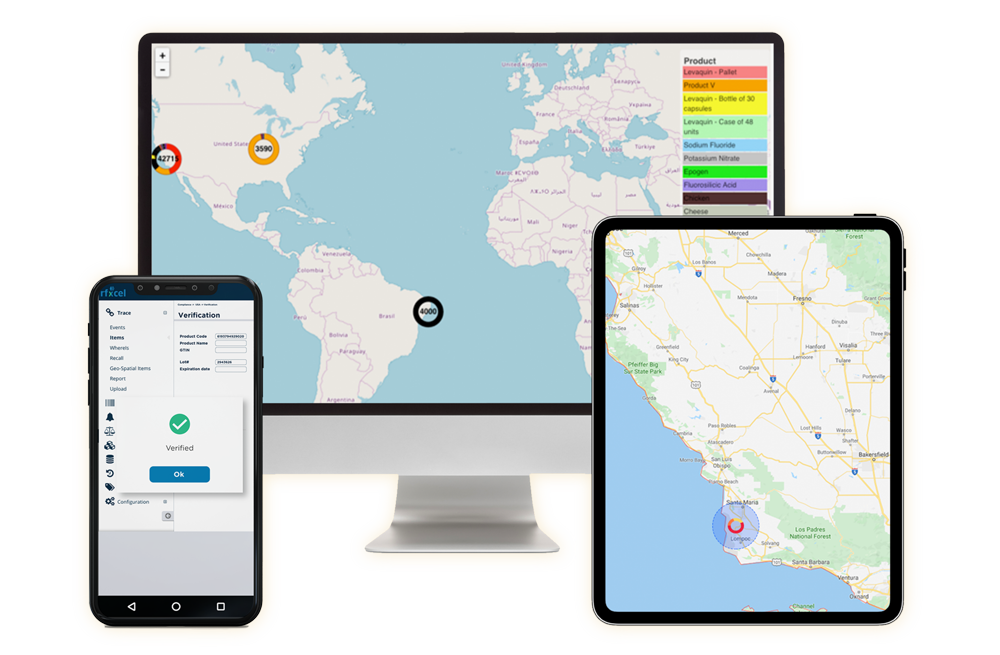
How rfxcel can help you with India iVEDA compliance
Our serialization and track and trace technology can help you maintain India compliance and safeguard your products. rfxcel’s expertise and technology also creates a digital supply chain that will transform your business value.
Our India support team can help you increase supply chain visibility
rfxcel has 20 years of experience increasing supply chain visibility. Our team in India will walk you through every step of the implementation process, ensuring full integration with our pre-configured, pre-validated compliance, serialization, and track and trace solutions.
You’ll also benefit from 24/7 growth-driven support. You’ll be assigned a dedicated rfxcel support team member who will resolve issues promptly and effectively
Secure your supply chain with rfxcel Traceability System
Our award-winning track and trace system, rfxcel Traceability System, helps you create a more scalable supply chain by allowing you to quickly add new features. It will also improve your supply chain with increased visibility. Contact us to learn how rfxcel helps companies simplify and accelerate their processes and comply with India iVEDA.
Your value beyond compliance with rfxcel
- Digitize and centralize your entire supply chain in one elegant, easy-to-use, intuitive platform
- Enhance performance and reduce processing time in multiple languages
- Leverage IoT technology to gain new real-time insight into and control of your supply chain
- Trace your products forward and backward
- Maintain historical information for a batch or individual product with continuous review and supply chain optimization
- Drive growth by lowering costs and risks, eliminating inefficiencies, and tracking all activity anywhere in your supply chain, including among suppliers, distributors, and 3PLs
- Integrate with any trading partner

