
Canada Track & Trace Requirements
In a February 2022 publication entitled “Roadmap to the Implementation of GS1 DataMatrix Barcodes on Pharmaceuticals in Canada,” GS1 wrote that Canada was actively working to require 2D DataMatrix codes on pharmaceutical products. GS1 Canada’s Pharmacy Board has approved a roadmap for expectations and requirements for manufacturers, distributors and pharmacies.
About Canada Track and Trace Requirements
Canada’s proposed regulations will apply to all categories of pharmaceuticals: prescription drugs, behind-the-counter drugs, over-the-counter (OTC) drugs, and natural health products approved to be marketed under the country’s Natural Health Products Regulations.
The proposed deadline to have “system-wide capabilities” in place is 2025. At that time, supply chain stakeholders will have to be able to scan, store, and process DataMatrix codes that contain a Global Trade Item Number (GTIN), a lot number, and and expiry date. Serial numbers are optional.
The roadmap also discusses “a collaborative approach to request that Health Canada introduces regulations to harmonize pharmaceutical barcoding requirements in alignment with regulators around the globe.”
At present, the roadmap gives manufacturers until Dec. 31, 2023, to have their system-wide capabilities in place; distributors have until Dec. 31, 2023, and pharmacies have until Dec. 31, 2025.
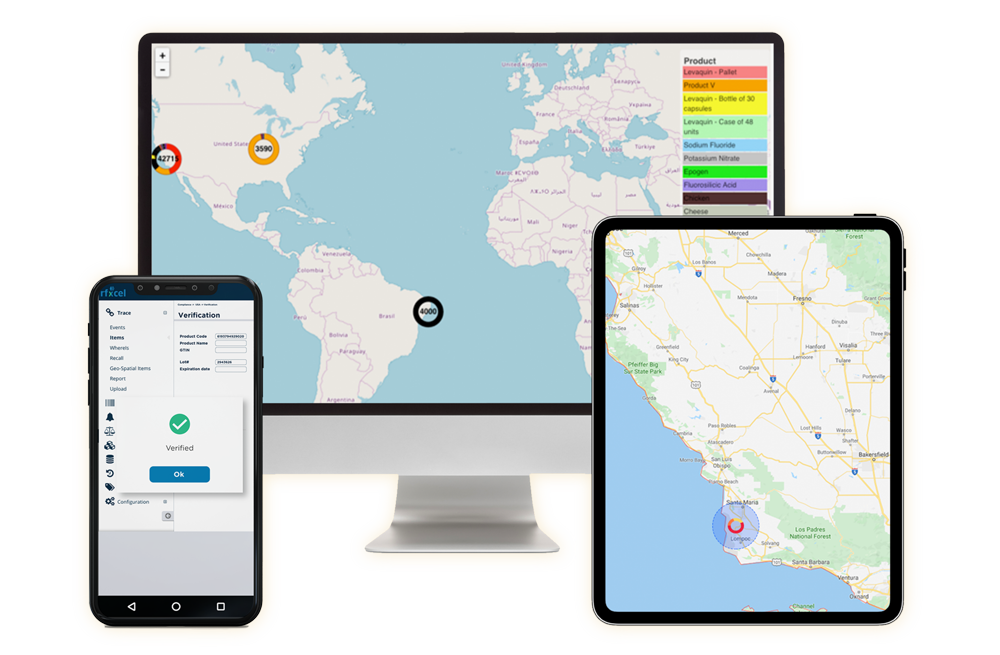
rfxcel Meets Canada’s Serialization & Traceability Requirements
rfxcel Is Your Partner for Canada Compliance
Our team will partner with you to meet Canadian requirements and transform business value with a transparent digital supply chain. With dedicated track and trace solutions from rfxcel, you can safeguard your products and brand reputation by ensuring all items are legitimate, marked, and fully compliant with Canadian law.
rfxcel’s Team: Compliance, Visibility & Support
rfxcel’s team will help you achieve and maintain Canada compliance by bringing end-to-end visibility to your supply chain.
Growth-Centered Solutions
rfxcel will develop a fully validated implementation plan tailored to your needs and designed specifically for Canada. Our systems operate in local languages, local currency, and processes, easing the compliance burden for companies in any industry. Our goal is to increase company value through insightful and precise data by automating and digitizing workflows.
Growth-Driven Support
rfxcel provides 24/7 support to all our customers. We assign a dedicated support team member to our customers so we can quickly and thoroughly resolve any issues that arise.
Secure Your Supply Chain with rfxcel Traceability System
Our award-winning Traceability System is a fully customizable platform that simplifies and accelerates compliance and increases supply chain visibility no matter where you do business. It also integrates seamlessly with your existing systems and enhances scalability by letting you add new features quickly.
Find out more about our Canada compliance solutions.
Your value beyond compliance with rfxcel
- Digitize and centralize your entire supply chain in one elegant, easy-to-use, intuitive platform
- Enhance performance and reduce processing time in multiple languages
- Leverage IoT technology to gain new real-time insight into and control of your supply chain
- Trace your products forward and backward
- Maintain historical information for a batch or individual product with continuous review and supply chain optimization
- Drive growth by lowering costs and risks, eliminating inefficiencies, and tracking all activity anywhere in your supply chain, including among suppliers, distributors, and 3PLs
- Integrate with any trading partner

