
Brazil ANVISA Track & Trace Requirements
Pharmaceutical companies manufacturing in or importing to Brazil are required to follow the regulations in the National Medicine Control System (SNCM), as described in Law No. 13.410.
There are key deadlines in April 2022. rfxcel will ensure you’re 100 percent compliant and that you remain compliant today, tomorrow — always.
About Brazil Track & Trace Requirements
The SNCM requires unit-level serialization, track and trace, and monitoring environmental conditions as products move through the supply chain. Every item must be visible at the unit level, and all products must have a GS1 2D DataMatrix code with the following information:
- Global Trade Item Number (GTIN)
- 13-digit ANVISA Medicine Registry Number
- Unique 13-digit serial number
- Expiration date (in the MM/YY format for human-readable form)
- Lot/batch number (up to 20 alphanumeric characters)
There are three key requirements for April 2022:
- All prescription medicines must be serialized.
- All manufacturers and importers must have a “serialization plan” in the SNCM portal.
- All supply chain stakeholders must submit product event reports to the SNCM.
rfxcel can help you meet these Brazil ANVISA serialization and track and trace requirements.
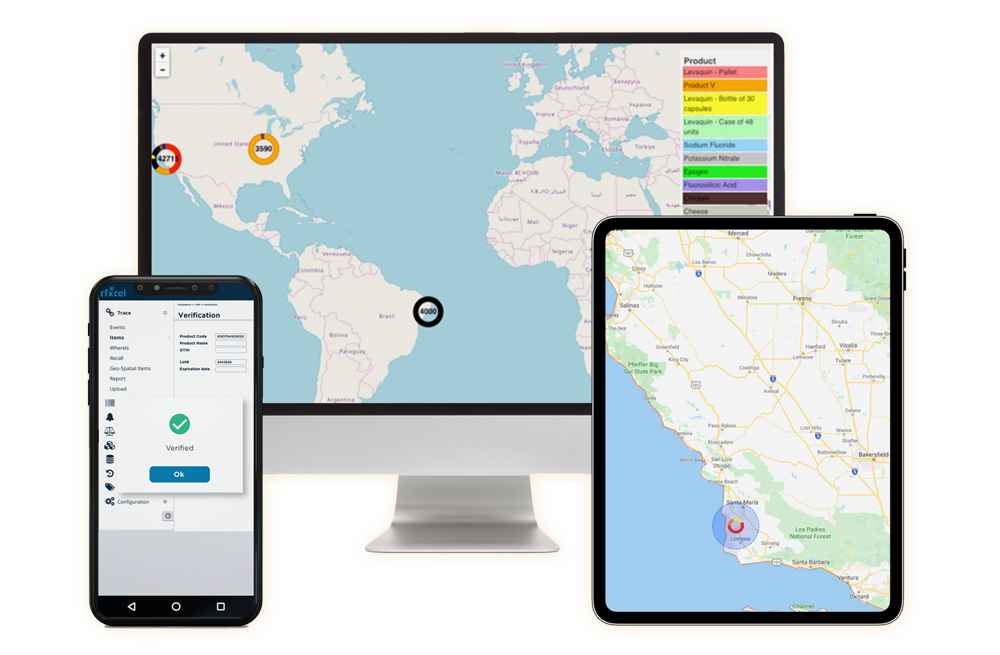
rfxcel Meets ANVISA Serialization & Traceability Requirements
rfxcel Is Your Partner for Brazil ANVISA Compliance
Our team will partner with you to meet Brazil ANVISA requirements and transform business value with a transparent digital supply chain. With dedicated track and trace solutions from rfxcel, you can safeguard your products and brand reputation by ensuring all items are legitimate, marked, and ANVISA-compliant.
rfxcel’s Brazil Team: Compliance, Visibility & Support
rfxcel’s team in Brazil will help you achieve and maintain ANVISA compliance by bringing end-to-end visibility to your supply chain.
Experienced Sao Paulo Team
Our Sao Paulo team has more than 15 years of experience increasing supply chain visibility. This local team will walk you through every step of the process to ensure full integration. rfxcel is prioritizing assisting Brazilian companies to meet compliance deadlines by providing our pre-configured and pre-validated solutions to simplify the implementation process.
Growth-Centered Solutions
rfxcel will develop a fully validated implementation plan tailored to your needs and designed specifically for Brazil. Our systems operate in the Portuguese and Spanish languages and use local currency and processes, easing the compliance burden for companies in any industry. Our goal is to increase company value through insightful and precise data by automating and digitizing workflows.
Growth-Driven Support
rfxcel provides 24/7 support to all our customers in Brazil. We assign a dedicated support team member to our clients so we can quickly and thoroughly resolve any issues that arise.
Secure Your Supply Chain with rfxcel Traceability System
Our award-winning Traceability System is a fully customizable platform that simplifies and accelerates compliance and increases supply chain visibility no matter where you do business. It integrates seamlessly with your existing systems and enhances scalability by letting you add new features quickly.
Find out more about our Brazil ANVISA compliance solutions today!
Full Guide to Brazil ANVISA Pharma Serialization and Traceability Regulations (Portuguese only)
Your value beyond compliance with rfxcel:
- Digitize and centralize your entire supply chain in one elegant, easy-to-use, and intuitive platform
- Enhance performance and reduce processing time in multiple languages
- Leverage IoT technology to gain new real-time insight into and control of your supply chain
- Trace your products forward and backward
- Maintain historical information for a batch or individual product with continuous review and supply chain optimization
- Drive growth by lowering costs and risks, eliminating inefficiencies, and tracking all activity anywhere in your supply chain, including among suppliers, distributors, and 3PLs
- Integrate with any trading partner

