

Serialization in Pharmaceutical Track and Trace is an industry standard operating procedure. All supply partners to pharmaceutical companies are asked to prepare lines, sites and infrastructure to meet complex regulatory requirements. rfxcel recommends strategic initiatives that are solutions for the entire manufacturing process. With its interconnected ecosystem rfxcel integrates with any of your partners and customers. rfxcel ensures your success.
Repackagers repack and relabel a product or a package for sale or for distribution. This also includes in-house repackaging departments within hospital networks. Repackagers have most of the same requirements that manufacturers and wholesale distributor do. rfxcel is allows you to disaggregate and reaggregate according to your packaging needs.
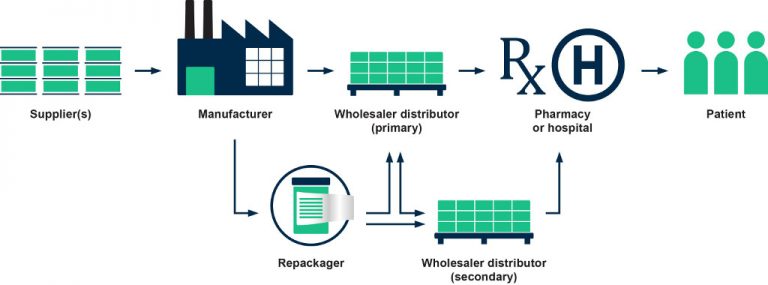
An effective compliance program has many benefits for wholesalers. With the help and expertise of rfxcel, pharmaceutical wholesalers can build internal monitoring and auditing into the compliance programs. rfxcel provides a framework for compliance to ensure that wholesalers stay within regulatory bounds.
Compliance regulations establishes a global pharmaceutical track and trace system that deters drug counterfeiting and ensures health. The rfxcel platform offers the ability to verify and process supply chain transactions. A flexible platform to serve your diverse supply chain community.

3PLs manage the transportation of goods between trading partner within the supply chain. rfxcel ensures that the information is properly communicated regarding product shipments.
Serialization and related track and trace regulations are required to do business in certain countries. rfxcel ensure that you meet these compliance requirements. rfxcel flexible system and industry engagement enables you to stay compliant regardless changes in the laws.