
Africa Track & Trace Requirements
Africa has the highest growth rate in the world. By 2100, the population will approach parity with Asia. Africa has also led the world in urbanization this decade. As of 2021, 609 million people lived in urban areas; this could reach 722 million by 2026. To keep pace with this growth, individual countries, Regional Economic Communities, and continental bodies are all working on regulating the pharmaceutical supply chain.
About Track and Trace Requirements in Africa
Compliance in Africa is a complex topic. We suggest starting with our blog series about the African supply chain for an overview.
For compliance in African countries, it’s important to follow developments in the eight Regional Economic Communities (RECs), individual countries, and the African Medicines Union. For example:
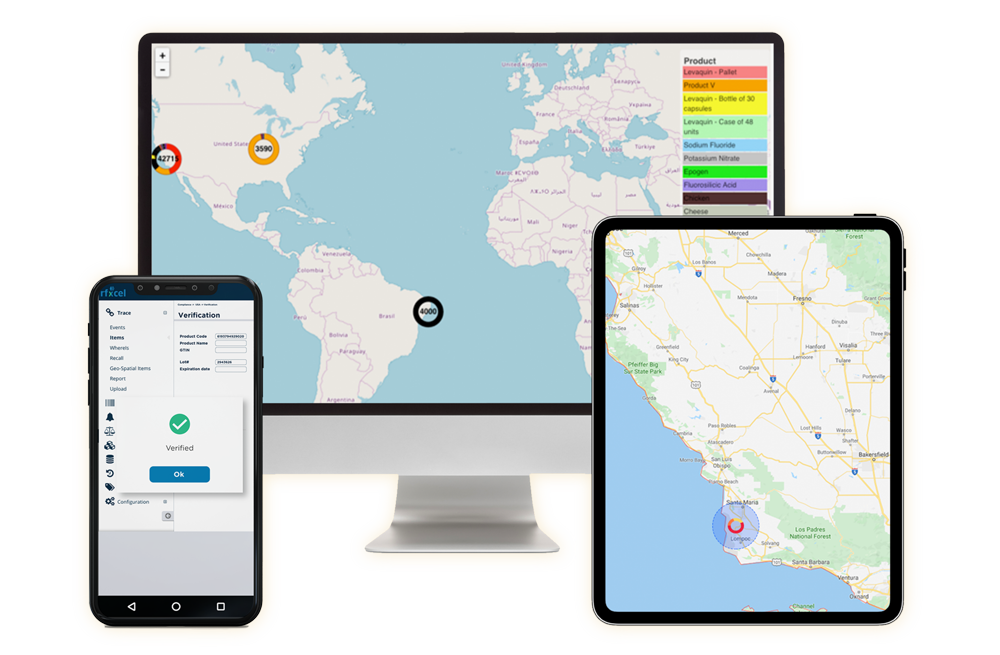
Algeria is a member of the Arab Maghreb Union REC and is expected to have its serialization requirements completed by 2023. In 2021, the government published barcoding regulations for products for human consumption, including medicines.
Ethiopia is a member of two RECs, the Intergovernmental Authority on Development (IGAD) and the Common Market for Eastern and Southern Africa (COMESA), and has published a timeline for its pharmaceutical regulations:
- Aug. 19, 2023: Data standard compliance (imported medicines)
- Aug. 19, 2023: Batch/Lot
- Feb. 19, 2024: Serialization (for certain medicines)
- Aug. 19, 2024: Batch/Lot
- Feb. 19, 2027: Traceability
Nigeria is a member of the Economic Community of West African States (ECOWAS) and the Community of Sahel-Saharan States (CEN-SAD) RECs. To date, it requires serialization for only antimalarials and antibiotics; however, in 2020 it announced a National Pharmaceutical Traceability Strategy and a 5-year roadmap.
Tunisia is a member of the Arab Maghreb Union, COMESA, and CEN-SAD RECs. It does not require serialization and has not announced plans to do so.
The African Medicines Agency (AMA) was established in January 2015. To date, 30 countries have backed it, but there are notable holdouts (e.g., Nigeria, South Africa, Kenya). Broadly, the AMA’s goals are:
- Registration and marketing of health technologies
- Granting manufacturing and distribution licenses
- Conducting quality and safety inspection of health technologies and manufacturing facilities
- Authorizing clinical trials through an established National Ethics Committee or Institutional Review Board
- Overseeing appeals procedures through an established Administrative Appeals Committee
rfxcel Meets African Serialization & Traceability Requirements
rfxcel Is Your Partner for African Compliance
Our team will partner with you to meet regulatory requirements in Africa and transform business value with a transparent digital supply chain. With dedicated track and trace solutions from rfxcel, you can safeguard your products and brand reputation by ensuring all items are legitimate, marked, and fully compliant with all applicable laws.
rfxcel’s Team: Compliance, Visibility & Support
rfxcel’s team will help you achieve and maintain compliance by bringing end-to-end visibility to your supply chain.
Growth-Centered Solutions
rfxcel will develop a fully validated implementation plan tailored to your needs and designed specifically for African countries. Our systems operate in local languages, local currency, and processes, easing the compliance burden for companies in any industry. Our goal is to increase company value through insightful and precise data by automating and digitizing workflows.
Growth-Driven Support
rfxcel provides 24/7 support to all our customers. We assign a dedicated support team member to our customers so we can quickly and thoroughly resolve any issues that arise.
Secure Your Supply Chain with rfxcel Traceability System
Our award-winning Traceability System is a fully customizable platform that simplifies and accelerates compliance and increases supply chain visibility no matter where you do business. It also integrates seamlessly with your existing systems and enhances scalability by letting you add new features quickly.
Find out more about our African compliance solutions and visit our blog for more information, including the eight RECs and the push for continent-wide harmonization through the African Medicines Agency.
Your value beyond compliance with rfxcel
- Digitize and centralize your entire supply chain in one elegant, easy-to-use, intuitive platform
- Enhance performance and reduce processing time in multiple languages
- Leverage IoT technology to gain new real-time insight into and control of your supply chain
- Trace your products forward and backward
- Maintain historical information for a batch or individual product with continuous review and supply chain optimization
- Drive growth by lowering costs and risks, eliminating inefficiencies, and tracking all activity anywhere in your supply chain, including among suppliers, distributors, and 3PLs
- Integrate with any trading partner

